I have smoked ribs about four times in the last couple years. I’ve been fortunate enough to have my father’s MasterBuilt smoker. It is a box about the size of a dorm fridge, and can readily smoke two racks, each cut in half. It has electronic temperature management and is well insulated. I’m fond of the smoker, and totally committed to the ribs from it.
At the bottom of the smoker is a pan, like a porkpie hat upside down. MasterBuilt’s instructions say to put water in the pan. Other sources suggest putting something solid in the pan instead. The argument is that boiling water is around 212°F (205°F at my altitude), and that the smoker’s temperature will not rise above that. A very reasonable argument. Consequently, I have used bricks wrapped in aluminum foil in the bottom of the pan.
What does the thermal mass do, whether bricks or water? One idea is that it causes the temperature to be more even, especially for it to bounce back faster after the door has been opened. For this to work well the thermal mass should a) be quite conductive, and b) have a high specific heat. Conductivity makes the heat inside the mass available to the air in the smoker. High specific heat means that a modest amount of mass can hold lots of heat.
| Material |
Conductivity (W/m K) |
Specific Heat (kJ/kg K) |
| Brick | 0.8 | 0.9 |
| Water | 0.58 (but can convect) | 4.2 |
It is hard to beat water for specific heat, and convection within the water pool should make the effective conductivity much higher than the intrinsic conductivity.
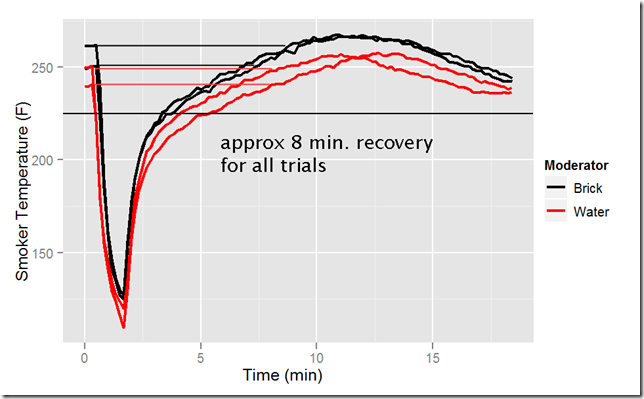
Which thermal mass provides faster temperature bounce back? It turns out they are functionally identical. The following figure shows four trials, two with water and two with brick moderator. During each trial I held the door open for one minute. There were differences in breeze and in ambient temperature (not analyzed). However, the chart shows that in all cases the temperature returned to its pre-opened temperature in the same amount of time, around 8 minutes. Rebound time is not much affected by the choice of moderator.
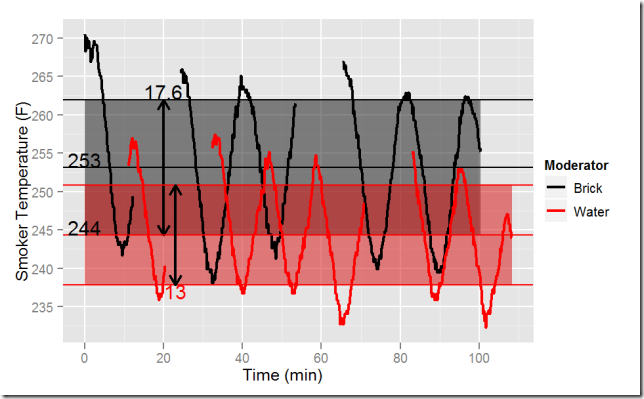
Which thermal mass provides more even temperature? This is harder to measure because I opened the door for the rebound test. Nevertheless, by removing the open-door data I can see the thermostat’s temperature management wave, and estimate the variability.
The next figure overlaps these two sets. In both sets the temperature swing is quite high, either 13°F or 17.6°F as measured by twice the sample standard deviation. Water provides more even temperature, but one that still varies quite widely.
The chart reveals the tie-breaker fact: the average water temperature is almost 10°F closer to the thermostat’s set point. The set point is 225°F, as recommended for ribs. The thermocouple was positioned at the top 1/4 of the cooking chamber, in the center front/back and left/right. This is where the top slab of ribs goes.
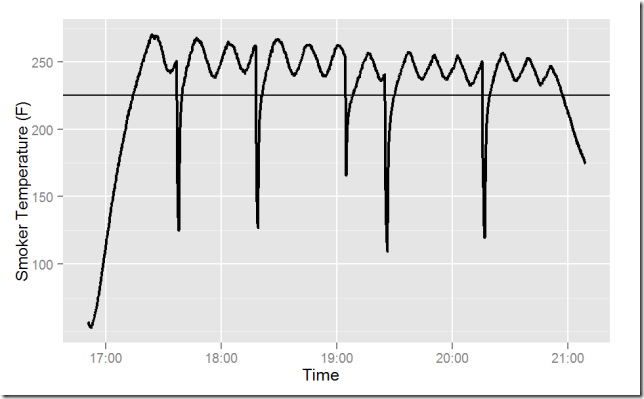
Presumably the bottom half of the cooking chamber is cooler; rotating the food top-to-bottom may help. The full data record is plotted in the final chart. The warm-up and cool-down bracket the data set. The record started with brick moderator, until about 19:05, when I replaced the brick with boiling water. The deep dips in temperature come from opening the door.
The next experiment is to instrument the box at several heights in the column, but that is low priority. Spring is coming and pruning must be attended.